Jennifer Fiegel explains motivation for research in chronic infections
Research Interests
Treatments for Persistent Bacterial Infections in the Lungs
Bacterial biofilms are communities of bacteria that form when the bacteria adhere to a surface in an aqueous environment and begin to excrete a protective polysaccharide matrix that holds the biofilm together. Bacterial biofilm formation in the lungs is a major concern for humans who have cystic fibrosis (CF), are immunocompromised, or are on mechanical ventilation. While aggressive therapies combining multiple antibiotics with mucus modulators alleviate some acute symptoms caused by biofilm infections in the lungs, the infection is not completely eradicated due to the protection afforded by biofilm formation. To locally treat persistent bacterial infections in the lungs, particularly due to Pseudomonas aeruginosa infection, our group has identified new combination therapies that work synergistically to eliminate biofilms by dispersing bacteria from within the biofilm, taking advantage of the greater susceptibility of dispersed bacteria to enhance the effectiveness of traditional antibiotics. Support for this work has been provided by the NIH and PhRMA Foundation.
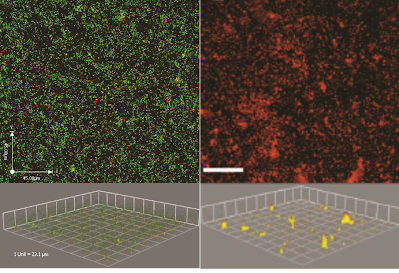
To support our work in characterizing bacterial biofilms, we have developed several new computer algorithms to enhance analysis of 3-D confocal microscopy images (see 'Source' tab).
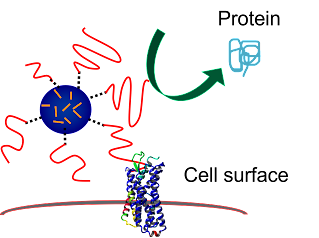
In our latest work supported by the NSF, we are using knowledge gained about how bacteria are able to bind to human tissue so they can persist in the lungs, and using that knowledge to develop pathogen-mimicking coatings to target drug carriers to human lung cells. This work is done in collaboration with a pharmacology expert, Prof. David Roman in the College of Pharmacy, and a receptor-ligand molecular dynamics expert, Prof. Blake Mertz from VCU.
|
|
Treatments for Chronic Wound Infections
We are now using our knowledge and expertise in tackling persistent infections to treat chronic skin infections. This work focuses on designing improved delivery systems for antimicrobials and pain medication, in collaboration with Prof. Nicole Brogden in the College of Pharmacy. We aim to develop patient-friendly treatment approaches using controlled-release hydrogels and microparticles for long term delivery, and aerosolizable systems to reduce pain associated with administration.
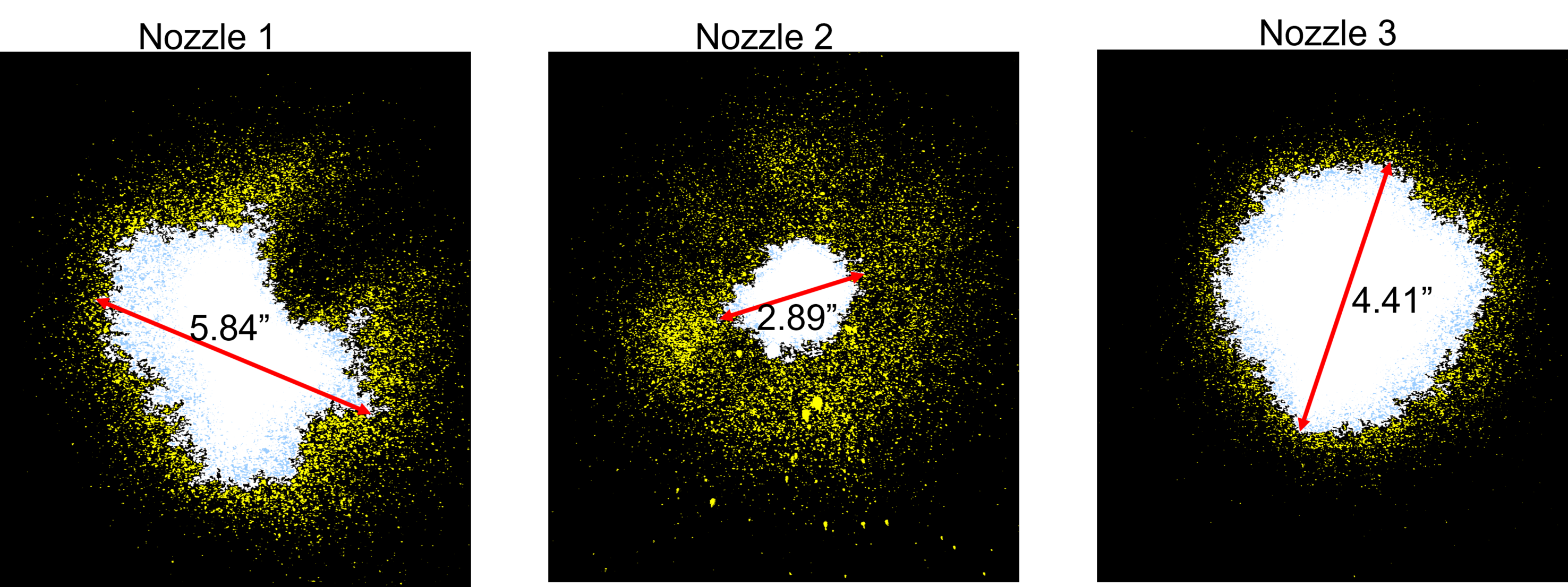
Spraying a thermoreversible hydrogel
Synthetic Mucus to Study Biointeractions
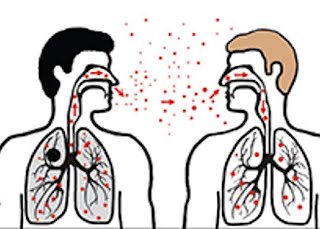
We have ongoing work that is focused on the development of mucus simulants that match the chemical composition, bulk physical properties, and surface properties of native mucus. These mucus simulants facilitate exploration of a wide range of mucus properties, which can easily be tailored using a synthetic approach. Our focus on controlling the surface properties of the fluids is particularly unique in our approach, enabling us to study lung relevant surface phenomena. Thus the mucus simulants have been applied to better understand the function of surfactant on viscoelastic gels, such as found in the large, conducting airways of the lungs, and to characterize the transport properties of aerosols that deposit on the surface of the lungs. We have also used mucus simulants to study the generation of droplets during simulated coughs (i.e. exhaled droplets that transmit infectious diseases between people, such as in COVID-19, tuberculosis, measles, and influenza).

|
|
|
Aerosol Interactions with Lung Fluids
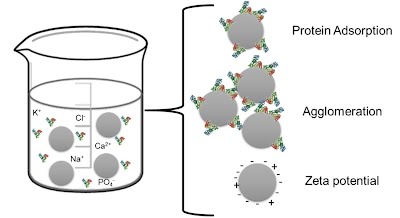
When aerosols deposit in the lungs, they interact first with the lung fluids. These initial interactions have a significant impact on the fate of the aerosols, controlling their subsequent interactions with the mucosal surface and lung tissues. We study a variety of phenomena related to these interactions, including 1) how protein adsorption to aerosol particles impacts their surface properties, stability in solution, and uptake properties; 2) how surfactants and mucus viscoelastic behavior may inhibit or enhance the spreading of drugs and drug carriers on the lung fluid surface; and 3) how inhalable nanoparticles may disrupt the normal functioning of lung surfactant.
|
|